Calcium And Oxygen Ionic Compound
Calcium oxide Formula
Calcium oxide Formula
Calcium oxide, as well known as quicklime, is a substance largely used in chemical industry equally intermediary in the cement and concrete product and also in the soda ash synthesis procedure.
Formula and structure: Calcium oxide chemic formula is CaO and its molar mass in 56.0774 chiliad mol-ane. The molecule is formed by the calcium cation Ca+2 and the oxygen anion O-2, which form a ionic bond. The calcium oxide ionic lattice is cubic and similar to NaCl lattice, with an ion surrounded by 6 contrary charge-ion. Its chemical structure can be written as below, in the common representations used for organic molecules.
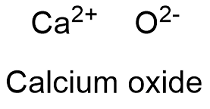
Occurrence: Calcium oxide is establish in nature as function of mineral Lime, which is a material composed past calcium, magnesium and aluminium carbonates, oxides and hydroxides.
Grooming: Calcium oxide is generally obtained from the lime through heating until the decomposition of material into CaO and COii:
CaCO3(s) → CaO(south) + CO2(southward)
Physical properties: Calcium oxide is an odorless, white to yellowish, gray or brown granular powder. Its density is 3.34 g mL-1. The melting and boiling points are 2613 ºC and 3850 ºC, respectively. It is soluble in water, reacting to form calcium hydroxide. Calcium oxide is insoluble in methanol and ethyl ether.
Chemical properties: Calcium oxide has a vast chemic reactivity. It tin react in many unlike ways, ii examples are:
Calcium oxide is used to obtain edifice material because when it is mixture with water can reacts to course calcium hydroxide, which rapidily forms a harden textile:
CaO + H2O → Ca(OH)2
It tin can besides react with fossil sources to produces calcium carbide:
two CaO(southward) + 5 C(due south) → 2 CaC2 (s) + COtwo (g)
Uses: Calcium oxide is very used in the production of edifice materials as cement and physical, similar to other calcium compounds as calcium sulfate. Moreover, calcium oxide is used in the manufacturing and production of substances equally soda ash, bleaching liquids, dry and desiccants and calcium compounds. It is as well used to produce fertilizers and as condiment in the water treatment/purification.
Wellness furnishings / condom hazards: Calcium oxide tin can cause severe eye and mucous irritantion. It is extremely toxic by ingestion and is corrosive to the pare. When large quantities of calcium oxide are dissolved in water, the resulting reaction is highly exothermic. It is corrosive and can react violently with ethanol, phosphorus pentoxide and acids.
Calcium And Oxygen Ionic Compound,
Source: https://softschools.com/formulas/chemistry/calcium_oxide_formula/431/
Posted by: dorseydouncestably.blogspot.com


0 Response to "Calcium And Oxygen Ionic Compound"
Post a Comment